Exovent: a new development from old technology
Few contemporary anaesthetists imagining negative pressure ventilation would
picture a modern, lightweight, torso-only device, and fewer still would imagine it
being described as “extremely pleasant” by a wide-awake subject who was able
to eat, drink and talk freely during exhalation (Figure 1). Yet, this is the Exovent
experience, as published last month in
Anaesthesia journal [1]. Unlike its ‘iron
lung’ predecessors, the Exovent can deliver the full features of negative pressure
respiratory support on a standard hospital bed. Continuous negative extrathoracic
pressure (CNEP) delivers the negative pressure equivalent of CPAP, and negative
pressure ventilation (NPV) can be augmented with negative end-expiratory
pressure (NEEP), the negative pressure equivalent of PEEP.
From the iron lung to positive
pressure ventilation
True collaboration between doctors and engineers is the
backbone of medical device development; the first effective iron
lung was designed by the Drinker brothers, one a physiologist
and the other an engineer. This template was used to treat
pneumonia as well as to save tens of thousands of poliomyelitis
victims (Figure 2). However, these cumbersome 300 kg, 2 m
long devices were largely abandoned when small positive
pressure ventilators were introduced in the mid-20th Century.
Though it was recognised that positive pressure ventilation (PPV)
would reduce cardiac output and require paralysis, sedation,
and tracheal intubation, these were considered an acceptable
price to pay. Seventy years later, we are increasingly aware of
ventilator-associated lung injuries cause by PPV and ventilator-associated
pneumonia caused by intubation.
The physiology of NPV
The physics of driving gases down the trachea towards the
alveoli through distensible airways produces very different
stresses to the lung microstructure and patterns of alveolar
expansion compared with gas being drawn into the lungs by
the alveolar distension generated by negative pressure. The
patchy atelectasis seen with PPV may result from distension of the proximal alveoli compressing the small airways of more distal
lung segments. The more evenly distributed forces generated by
NPV may explain the rarity of pneumothoraces. In essence, NPV
mimics ‘natural’ breathing more closely.
Applying extrathoracic negative pressure efficiently to healthy
humans or animals affects lung volumes and gas exchange in
a similar manner to positive pressure. Increasing ‘background’
inspiratory pressure using CNEP drives similar increases in
the functional reserve capacity (FRC) as CPAP, and equivalent
NPV and PPV inflation pressures produce similar tidal volumes.
However, when animals are ventilated after lung damage from
saline lavage or pulmonary artery oleic acid infusion, NPV
produces better oxygenation, less atelectasis (Figure 3), less
alveolar oedema, and less inflammation than PPV [2]. COVID-19
pneumonia brings the added concern that PPV stimulates the
expression of ACE2, the SARS-Cov-2 virus receptor [3].
Positive pressure and negative pressure also have different
physiological impacts on the circulation. Raised intrathoracic
pressures during CPAP and PPV may cause an ≈ 20% fall in
cardiac output by impeding systemic venous return, leading to a
smaller ventricular stroke volume. PPV may also have an impact
on the pulmonary microcirculation by compressing acinar vessels
and shunting blood away from aerated alveoli. CNEP or NPV do
not produce detectable haemodynamic sequelae.
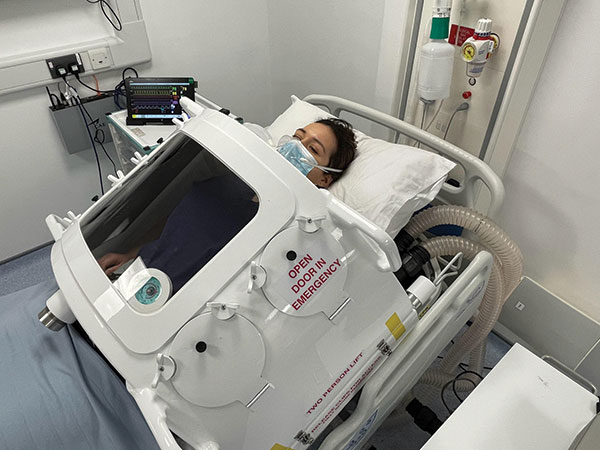
Above: Figure 1. A volunteer in an Extovent
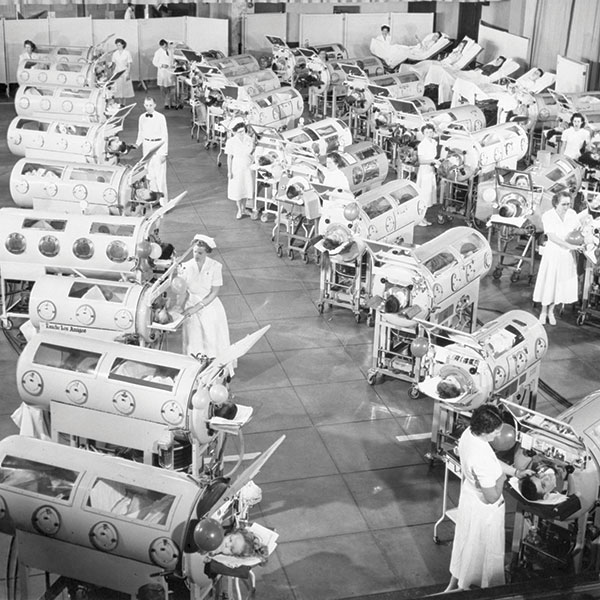
Above: Figure 2. Iron lungs in a polio ward
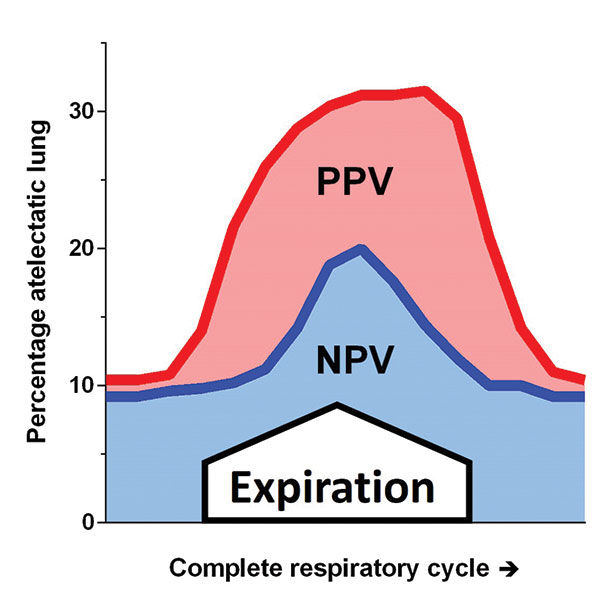
Above: Figure 3. Percentage of atelectatic lung during positive and negative pressure ventilation in surfactant depleted rabbits, adapted from reference [2]
Building a small but effective NPV device
There are four basic NPV designs: whole-body tanks (‘iron lungs’);
‘wrap’ and ‘shell’ cuirasses; and torso-only tanks such as the
Exovent (Figure 4). The efficiency of iron lungs varied between
models, but slowly rising inspiratory pressures were difficult to
avoid with the then-available suction pumps acting on large tank
volumes, limiting their capacity to generate high tidal volumes.
In ‘wrap’ cuirasses, anorak-type material is laid onto a frame over
the patient’s torso, and sealed below the axillae and at the hips.
Unfortunately, they lose efficiency at low pressures because the
material balloons in and out with every breath, and air leaks are
difficult to prevent at higher pressures. ‘Shell’ cuirasses are light
and portable, and seal directly onto the anterior chest, abdomen,
and lateral rib cage. Applying suction reduces the intrathoracic
pressure, but also pulls the shell edges down more firmly, risking
restricting diaphragmatic and thoracic wall movement.
The Exovent base with its own internal mattress is placed on
a bed, the cover is placed over the torso and arms, and the
neoprene neck (hyperboloidal) and hip seals fitted. Its chamber
is larger than a cuirass but much smaller than a whole-body tank,
which allows the pump to generate almost square inspiratory
pressure waves. Members of the Exovent development team
(a volunteer group of engineers, doctors and nurses) found it
comfortable to use supine at 30° head-up, or prone. It did not
produce dyssynchrony so long as the volunteer was instructed to
relax and not ‘fight it’. Just -5 cmH2O of CNEP increased the FRC
by over 5 ml.kg-1, and less than -4 cmH2O of NPV was sufficient
to generate resting tidal volumes of 11.4 ml.kg-1 (Figure 5). This
compares with typical chamber pressures of -20 to -40 cmH2O for
most previously-reported NPV devices.
What clinical contributions could Exovent make?
The Exovent allows healthy adults to receive the negative pressure
equivalents of CPAP, and ventilation plus PEEP, comfortably. The
enclosure can be removed quickly by two people if needed,
and the window and self-sealing portholes allow for clinical
monitoring and undertaking procedures. Despite fears to the
contrary, users have not found the chamber claustrophobic.
This may be because they can move relatively freely inside and
can easily breach the seals with their hands, either producing a
momentary pressure drop or releasing the vacuum completely.
We have not yet trialled the Exovent in patients, but this is
planned. However, the extensive history of NPV suggests that
it may provide a useful tool alongside conventional therapies
in treating people with COPD, pneumonia including COVID
19, and neuromuscular weakness. In particular, we wonder if a
key advantage may be the ability to move patients seamlessly
between CNEP and NPV without the need for tracheal
intubation, as even relatively high extrathoracic pressures remain
comfortable. This contrasts with the intolerance of pressures
sometimes required for CPAP or conventional non-invasive
ventilation, which may then require tracheal intubation. This may
offer a benefit to patients with a ceiling of treatment.
Finally, there are resource considerations. A UK version
is anticipated to cost approximately £8000, considerably
cheaper than conventional positive pressure devices,
and we aim to produce a low-cost version for low and
middle income countries for less than £500. It also has
the potential to reduce oxygen usage as it is powered
by electricity (potentially including batteries), so
patients will only need facemask or nasal oxygen. It is an
ambition of the Exovent charity (1189967) to improve
access to negative pressure ventilation globally.
Malcolm G. Coulthard
Honorary Consultant Paediatric Nephrologist
Great North Children’s Hospital, Newcastle upon Tyne
Jan van Egmond
Clinical Physicist, Laboratory of Anaesthesia Research,
Radboud University Medical Center, Nijmegen,
Netherlands
Anil Patel
Professor of Anaesthesia and Consultant Anaesthetist
Royal National ENT and Eastman Dental Hospitals,
UCLH, London
and the Exovent Team
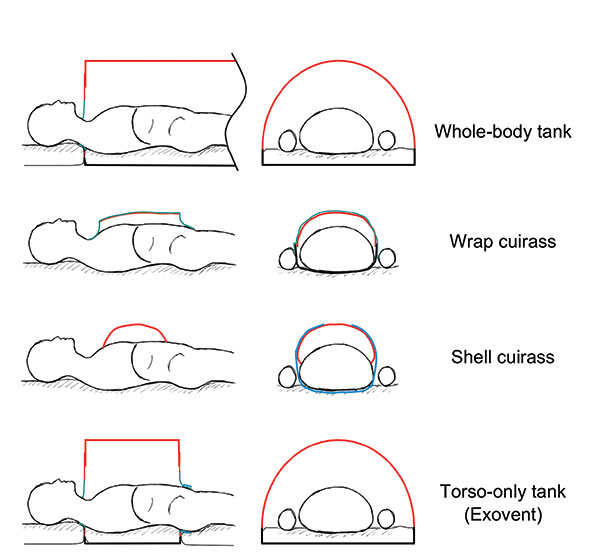
Above: Figure 4. Types of negative pressure ventilator chambers. Red - rigid material; green – cloth or flexible material; blue - velcro band
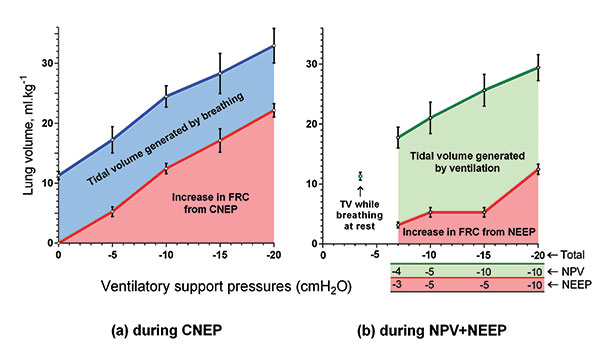
Above: Figure 5. Tidal volumes during NPV and NEEP, and increases in FRC during CNEP generated by the Exovent in six healthy volunteers, from reference [1]. Error bars = 1 SD
References
- The Exovent Development Group. Exovent: a study of a new
negative-pressure ventilatory support device in healthy adults.
Anaesthesia 2021; 76: doi:10.1111/anae.15350.
- Grasso F, Engelberts D, Helm E, et al. Negative-pressure ventilation:
better oxygenation and less lung injury.
American Journal of
Respiratory and Critical Care Medicine
2008; 177: 412-8.
- Huang S, Kaipainen A, Strasser M, et al. Mechanical ventilation
stimulates expression of the SARS-Cov-2 receptor ACE2 in the
lung and may trigger a vicious cycle. Preprints 2020; doi:10.20944/preprints202005.0429.v1.