Standardisation, syringe labelling and prefilled syringes
‘Medication Without Harm’ is the WHO third Patient Safety Challenge [1]. Much of the
knowledge is available, but needs to be consistently implemented. WHO’s three targets -
high risk medications, polypharmacy and transitions of care - are what anaesthetists do all the
time. Martin Bromiley, chair of the Clinical Human Factors Group, says “Standardisation has
been shown to be an effective mechanism for reducing human error in complex processes or
situations” [2]; medication processes are an area ripe for standardisation.
Labelling
The first medication standardisation in UK anaesthesia was
introducing standard user-applied syringe labels in 2003, before
which at least six different coloured label systems were in use [3,
4]. The specialty recommended standardisation using the existing
Australian/ New Zealand/ USA labelling standards [5], and a survey
of Association Linkpersons 12 months later found that more than
90% of hospitals were using them without any serious incidents
during the change. This was a notable speciality-led achievement,
with no lengthy regulation from the Department of Health or
MHRA.
Label positioning
In 2007 the National Patient Safety Agency (NPSA) standard
operating procedure for preparing injectable medicines advised
labelling the syringe only after filling, not before [6]. This is
logical as a label on an empty container can never be correct.
It is consistent with other labelling practice, for example many
serious incidents of incorrect blood samples for cross matching
have occurred when the name label was placed on an empty
sample tube [7]. The European Board of Anaesthesiology
recommendations state: the syringe should be labelled
immediately after filling and before leaving the operator's hand;
the label should be matched with the ampoule; this should be done one medication at a time [8]. In a recent survey 61% of
anaesthetists labelled syringes after filling, 21% before, and 18%
had no standard process [9].
The syringe should be labelled so that the syringe contents can
be identified before the clinician picks up the syringe. It is best
practice to stick at least one label longitudinally along the barrel
of the syringe so it can be read while the syringe is on the work
surface. Similarly, syringes should always be placed oriented
sideways so that they can be read easily. Standardised work trays
using this orientation have been shown to reduce medication
incidents [10].
A syringe label may be orientated either ‘left handed’ (nozzle
pointing right) or ‘right handed’ (nozzle pointing left (Figure
1). Standardisation to ‘right handed’ is recommended, as this
conforms with the orientation of syringe driver pumps, and
labelling of prefilled syringes.
Prefilled syringes
When in the 1990s AstraZeneca produced both 1% and 2%
propofol in prefilled syringes with a recognition tag in the flange
to create a safety identity link to the Diprifusor syringe driver
[11], anaesthetists thought that this the way all our drugs would
be supplied in 10 years time. However, the specialty failed to grasp the initiative despite the NPSA recommending
‘purchasing for safety’ policies. There is probably no other
healthcare area where so many human factor errors can be
completely removed as with the adoption of prefilled syringes.
Astonishingly, the NHS Specialist Pharmacy Service that
advises hospitals on medicines purchase has no reference to
human factors in their procurement overview [12].
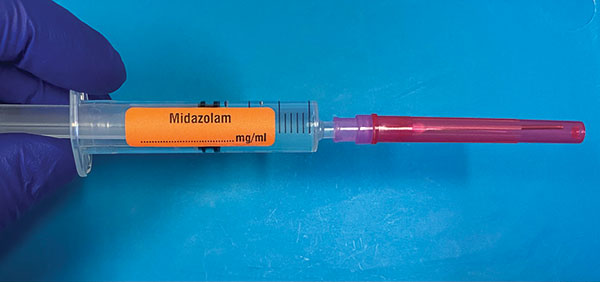
Figure 1. Left Handed Syringe
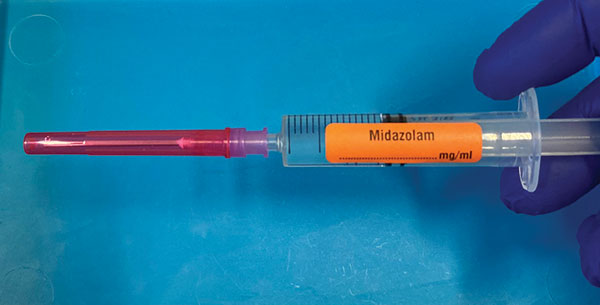
Right Handed Syringe
All drugs used in routine anaesthesia can now be supplied
in prefilled syringes. Besides ensuring the correct contents,
they can have a tamper-evident facility [13]. Sterility is also
guaranteed; up to 6% of the syringes drawn up in operating
theatres have bacterial contamination [14].
The latest Royal Pharmaceutical Society medicines guidance
now includes a section for operating theatres [15]. The
overriding themes are that manipulation of medicines in
clinical areas should be minimised, and medicines should
be presented as prefilled syringes or other ‘ready-to-administer’
preparations wherever possible. Using prefilled
syringes permits the standardisation of drug concentrations
for medicines that require dilution. Notably the London
Nightingale Hospital pharmacy established a prefilled syringe
compounding area [16], saving nurses time while wearing
cumbersome PPE.
‘Wrongly prepared high-risk injectable medication’ used to be
a Never Event, but none were reported and it was removed
in 2015 [17]. NAP5 identified six ampoule labelling errors
associated with awareness during general anaesthesia [18],
but sadly this was never used to demonstrate the need for the
robust systemic barrier of prefilled syringes.
In 2020 the WHO World Patient Safety Day was dedicated to
health worker safety and proposed five goals for healthcare
organisations [19]. One was ‘Prevent sharps injuries’, including
maximising the use of needle-less intravenous systems.
If intravenous access is already established, using safety engineered
devices such as prefilled syringes offers this
possibility. Preparing medicines with needles during transfers
can often be difficult (Figure 2), and the Association transfer
guidelines have recommended the preferential use of prefilled
syringes since 2009 [20, 21].
The specialty of anaesthesia has been left far behind in the
use of prefilled syringes. Of the 10 billion units of injectable
medicines sold annually, 28% are supplied in ready to
administer or prefilled preparations, yet in the acute sector
this is only true for 4%. Surely anaesthetists, as the specialists
in intravenous practice, should now be demanding this.
Anaesthetists are accustomed to the standardisation of
controls on anaesthetic machines and other equipment,
but many have their own foibles or quirks for arranging
the medication work surface. Standardisation is a powerful
safety tool, and particularly potent when working in teams; I
believe now is the time to introduce standardisation into perioperative
medication practices.
David Whitaker
Chair, Patient Safety Committee, European Board of Anaesthesiology
Manchester
February 2021: the Association are finalising draft guidelines on 'Syringe labelling and peri-operative medicines handling', which will shortly be made available for Member and Stakeholder consultation. Please watch this space.

Figure 2.
Illustration: ‘In Safe Hands’ The Medical Emergency Response Team aboard a CH47 Chinook above Southern Afghanistan, battles to save the life of an injured soldier by Stuart Brown. With permission, Skipper Press LTD, www.skipperpress.com
The original painting was donated to the Royal College of Anaesthetists by Colonel Peter F Mahoney OBE, TD, MSc, FRCA, L/RAMC Defence Professor
References
- Donaldson LJ, Kelley ET, Dhingra-Kumar N, Kieny M-P, Sheikh A. Medication Without
Harm: WHO's third Global Patient Safety Challenge.
Lancet 2017; 389: 1680-1.
- Union Européenne des Médicins Spécialistes. European Standardisation of the in-hospital
'Cardiac Arrest Call' Number - 2222, 2016. https://www.uems.eu/__data/assets/pdf_
file/0007/38644/Joint-Press-release-2222-20-9-2016-without-confidential.pdf (accessed
10/12/2020).
- Radhakrishna S. Syringe labels in anaesthetic induction rooms. Anaesthesia 1999; 54:
963-8.
- Christie IW, Hill MR. Standardized colour coding for syringe drug labels: a national survey.
Anaesthesia 2002; 57: 793-8.
- Birks RJS, Simpson PJ. Syringe labelling - an international standard. Anaesthesia 2003; 58:
518-9.
- National Patient Safety Agency. Promoting safer use of injectable medicines. A
template standard operating procedure for: prescribing, preparing and administering
injectable medicines in clinical areas, 2007. https://www.sps.nhs.uk/wp-content/uploads/2018/02/2007-NRLS-0434F-Promoting-safeSOP-template-2007-v1.pdf (accessed
1/12/2020).
- Bolton-Maggs PHB, Wood EM, Wiersum-Osselton JC. Wrong blood in tube - potential for
serious outcomes: can it be prevented?
British Journal of Haematology 2015; 168: 3-13.
- Whitaker D, Brattenbø G, Trenkler et al. The European Board of Anaesthesiology
recommendations for safe medication practice.
European Journal of Anaesthesiology
2017; 34: 4-7.
- ESAIC Academy. Webcast on Medication Safety, 2020. https://academy.esaic.org/esaic/2020/elearning-2020/288282/prof.teodora-orhidee.nicolescu.prof.benedikt.
preckel.doctor.jannicke.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asea
rch%3DMedication+safety (accessed 3/12/2020).
- Grigg EB, Martin LD, Ross FJ, et al. Assessing the impact of the anesthesia medication
template on medication errors during anesthesia: a prospective study.
Anesthesia and
Analgesia
2017; 124: 1617-25.
- Gray JM, Kenny GNC. Development of the technology for ‘Diprifusor’ TCI systems.
Anaesthesia 1998; 53: 22-7.
- Healthcare Safety Investigation Branch. Inadvertent administration of an oral liquid
medicine into a vein, 2019. https://www.hsib.org.uk/investigations-cases/inadvertent-administration-oral-liquid-medicine-vein/ (accessed 3/12/2020).
- Association of Anaesthetists. Safe drug management in anaesthetic practice, 2020.
https://anaesthetists.org/Home/News-opinion/News/Safe-Drug-Management-in-Anaesthetic-Practice (accessed 3/12/2020).
- Gargiulo DA, Mitchell SJ, Sheridan J, et al. Microbiological contamination of drugs during
their administration for anesthesia in the operating room.
Anesthesiology 2016; 124:
785-94.
- Royal Pharmaceutical Society. Professional guidance on the safe and secure handling of
medicines. Appendix C: Operating theatres - supplementary guidance, 2018. https://www.rpharms.com/recognition/setting-professional-standards/safe-and-secure-handling-of-medicines/professional-guidance-on-the-safe-and-secure-handling-of-medicines
(accessed 3/12/2020).
- Harchowal J. What a pharmacy team, 2020. https://twitter.com/jharchowal/status/1251424098317676546 (accessed 3/12/2020).
- NHS England. Revised Never Events policy and framework, 2015. https://www.england.nhs.uk/wp-content/uploads/2015/04/never-evnts-pol-framwrk-apr.pdf (accessed
10/12/2020).
- Pandit JJ, Andrade J, Bogod DG, et al. 5th National Audit Project (NAP5) on accidental
awareness during general anaesthesia: summary of main findings and risk factors.
Anaesthesia 2014; 69: 1089-101.
- World Health Organization. World Patient Safety Day Goals 2020-21, 2020. https://apps.who.int/iris/bitstream/handle/10665/334329/WHO-UHL-IHS-2020.8-eng.pdf (accessed
3/12/2020).
- Association of Anaesthetists of Great Britain and Ireland. Interhospital transfer, 2009.
https://anaesthetists.org/Portals/0/PDFs/Guidelines%20PDFs/Guideline_interhospital_
transfer_2009_final.pdf?ver=2018-07-11-163754-600&ver=2018-07-11-163754-600
(accessed 3/12/2020).
- Nathanson MH, Andrzejowski J, Dinsmore J, et al. Guidelines for safe transfer of the brain injured
patient: trauma and stroke, 2019: Guidelines from the Association of Anaesthetists
and the Neuro Anaesthesia and Critical Care Society.
Anaesthesia 2020; 75: 234-46.